Recently, Philips' 3T magnetic resonance system MR 7700 has received FDA 510(k) clearance. This MR system was first exhibited at RSNA2021 and appeared again at the annual meeting of the International Society of Medical Magnetic Resonance (ISMRM) held in London from May 7th to 12th this year.
MR 7700 imaging system features an enhanced gradient system for unmatched performance and precision, delivering Philips’ highest image quality to help improve diagnostic outcomes. The gradient intensity is as high as 65mT/m, and the peak conversion rate is 220T/m/s.
This system has a gradient chain of 65-20, and its XP gradient coil can increase the signal-to-noise ratio (SNR) by 35% and shorten the scanning time by 35%, enabling radiologists to complete more than 20% of the fMRI (functional MRI) scanning volume in neurological imaging, and more than 50% of the diffusion tensor imaging (DTI) direction, bringing more detailed high-resolution images.
Newest MR innovation from Philips integrates artificial intelligence, for example, non-contact patient induction and motion detection, to solve the problem of the surge in the number of patients currently faced by many medical institutions and enhance patient and staff experience,
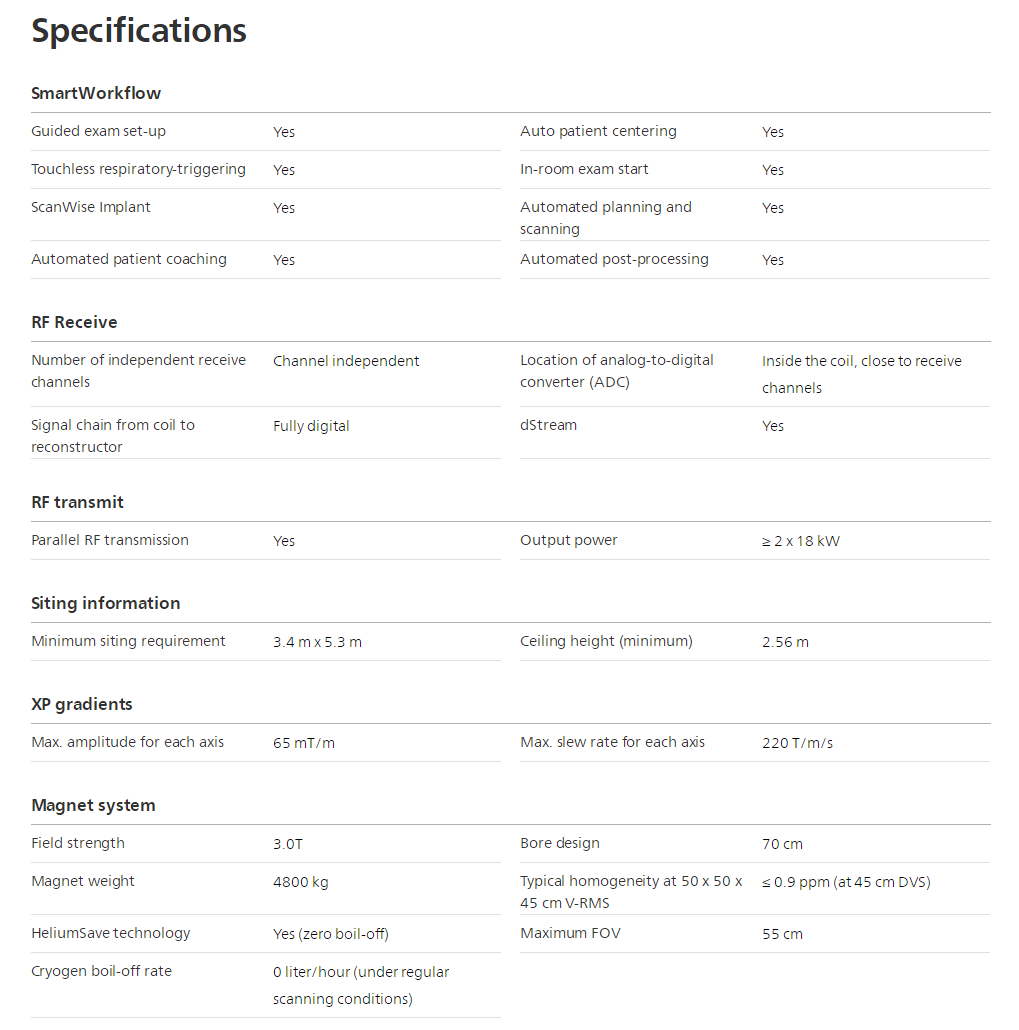
MR 7700 specifications
In addition to conventional proton magnetic resonance imaging and spectroscopy, MR 7700 has in-built MR protocols to combine proton imaging with imaging based on sodium, phosphorus, carbon, fluorine, and xenon [3]. This multi-nucleus capability makes MR 7700 suitable for enhanced anatomical and metabolic/functional imaging in areas such as neurology, pulmonology, and oncology to help deliver fast, and confident definitive decision-making.
For example, MR 7700 users can perform sodium (Na-23) imaging to measure the metabolic rate in the brain, as a biomarker for neurodegenerative diseases, or track stroke and ischemia, and even measure cartilage health. Phosphorus (P-31) spectroscopy can also be analyzed to measure energy metabolism in the brain for applications such as oncology or hemodynamic disorders.
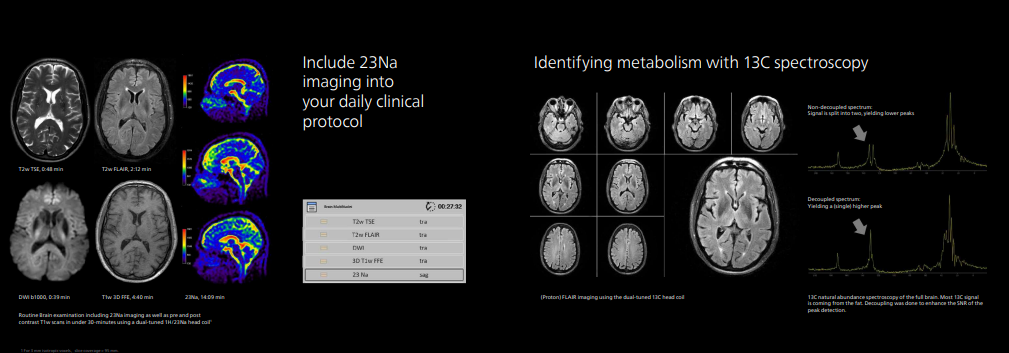
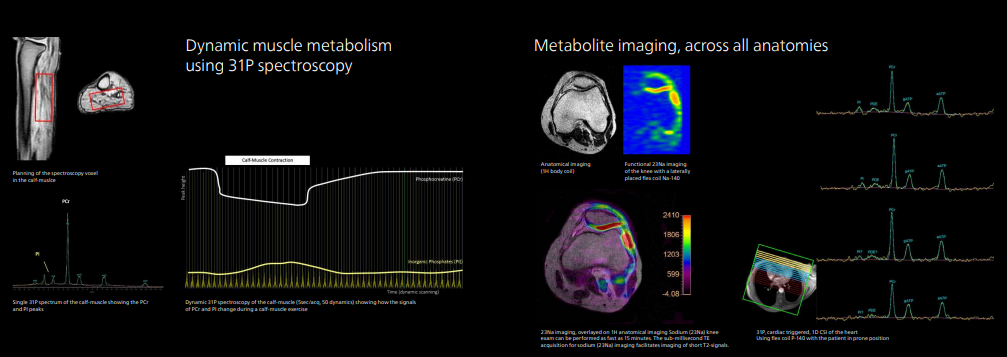
The XP gradient will contribute to neuroscience research projects without affecting workflow efficiency or patient comfort. Users only need to select a parameter in the conventional protocol on the interface, and they can more easily switch from the clinical protocol to the research protocol. The solution provides the necessary code for each protocol.
Philips is the first to bring multi nuclei to clinical practice with seamless integration into standard user interface of 3.0T dStream systems.
 English
English
 Русский
Русский